Dehydrated Evergreen,Evergreen Dehydrated Vegetables,Air Dried Evergreen,Air Dried Evergreen Vegetables Xinghua Lvwei Foods Co.,Ltd , https://www.lvweifoods.com
12017, Novartis's CTL-019 will be the world's first event to be approved for CRA-T therapy, which has exploded the entire circle of friends. This day, for people in the field of cancer immunotherapy, is undoubtedly very memorable, because it opens a new era of cancer treatment. Since 2013, Science magazine has listed "Tumor Immunotherapy" as the first breakthrough in the year. Since then, chimeric antigen receptor T cell therapy (CAR-T therapy ) has emerged as one of the shining stars. . In order to let more people understand the past, current status and future of CAR-T, an emerging anti-tumor technology, we have specially compiled the information and launched the CAR-T topic column to share with you the CAR-T "gossip". Those things about CAR-T. 
"Gossip" 1: Originally CAR-T received much attention from the little girl Emily
CAR-T therapy has received so much attention and expectation from scholars, doctors, patients, and investors around the world. It originated from a leukemia girl Emily and her magical anticancer experience.
Let's take a look at Emily 's magic story!
Emily is a little girl from California. She was unfortunately diagnosed with acute lymphoblastic leukemia (ALL) when she was 5 years old. Even more unfortunately, she accidentally infected her in the first round of chemotherapy, almost lost her legs, and her condition. Frequent recurrence. Just as doctors had nothing to do, scientists at the University of Pennsylvania decided to adopt a crazy, unprecedented treatment.
Miraculously, after this crazy therapy, Emily is still healthy and there is no cancer recurrence in five years. She can go to school, play, and learn piano. To this end, there is also a dedicated website describing her life in the fight against cancer for a few years: http://emilywhitehead.com/. As shown below: 
What magical way to cure this little girl?
The treatment process is like this:
1 The doctor first draws blood from Emily's body, extracts white blood cells from the peripheral blood, and then genetically engineers the white blood cells with modified HIV to attack them and reinject them into Emily.
2 The cancerous white blood cells in Emily began to decrease sharply, but these cells also attacked her body! Emily had a high fever for several days and even had an illusion. Some doctors told her family that she was only one-thousandth of the chance to live that night... It seems that this is a bold attempt.
3 But the miracle happened: the doctors used Emily to use a type of rheumatoid arthritis drug to stop the immune system from reacting strongly, and the drug did not protect cancer cells. Emily woke up on her 7th birthday and her body gradually recovered.
In fact, the magical treatment to cure Emily is the emerging anti-tumor technology we mentioned in this issue, CAR-T therapy.
"Gossip" 2 : What exactly is CAR-T ?
l Basic principle of CAR-T :
CAR-T , the full name is Chimeric Antigen Receptor T-Cell , chimeric antigen receptor T cell immunotherapy, in simple terms, genetically engineered patients' own T cells, plus chimeric antigen receptor (CAR), after The modified T cells recognize and attack tumor cells with specific antigens, and activate the body's own immune response to achieve anti-tumor effects. 
l About T cells:
As we all know, T cells are the most ferocious "hunters" in the immune system. The variable region (scFv) of the antibody single chain expressed by T cells in combination with the T cell signaling region is capable of regulating the function and specificity of T cells. They use their own receptors to sense cells with specific proteins on their surface (for tumor cells, tumor-associated antigens or tumor-specific antigens), then lock infected cells and cancer cells and kill them.
l About CAR molecules: 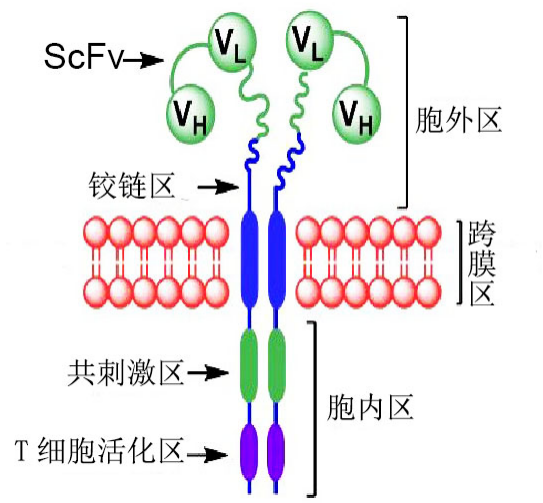
CAR generally consists of an antigen-binding/extracellular/transmembrane region and an intracellular signal region capable of activating T cells after binding to an antigen, as shown
Classic CAR structure diagram <br> Since 1989, Israeli immunologist Zelig Eshhar has fused the immunoglobulin scFv to the FcεRI receptor (γ chain) or CD3 complex (ζ chain) intracellular domain and introduced it into T cells to prepare Since the first generation of CAR-T, CAR-T has experienced more than 20 years of development.
CAR molecules can be roughly divided into five generations :
Generation I: CD3 molecule-mediated specific T cell activation;
Generation II: intracellular segments increase co-stimulatory factors and enhance cell killing activity;
Generation III: Simultaneous addition of two costimulatory factors to increase T cell proliferation and killing activity;
Generation IV: Integrate a variety of regulatory genes, such as suicide genes, cytokine release (such as IL-7, IL-15);
Generation V: Universal CAR-T against immune rejection and graft versus host disease. The figure below shows the structure of the 1-4 generation CAR.
At present, the second and third generations of CAR-T are relatively mature , especially the second generation, and the fourth and fifth generations of CAR-T are also being widely developed. 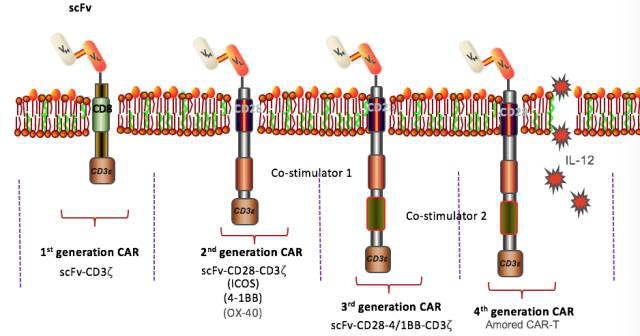
“Gossip†3 : How is CAR-T prepared and functioning?
As shown below:
1. Isolating T cells from a patient;
2. Bring the T cells with a "GPS navigation" (CAR molecule) that identifies the tumor. That is, using genetic engineering technology to add a chimeric receptor (essentially a fusion protein) to T cells that recognizes tumor cells, simultaneously activates T cells, and finally kills tumor cells;
3. Large-scale expansion of CAR-T cells in vitro to achieve therapeutic levels;
4. The in vitro cultured CAR-T cells are returned to the patient;
5, CAR-T plays a role in patients; genetically modified CAR-T cells expressing chimeric antigen receptors will not only kill cancer cells, but also begin to divide, creating a huge army of cancer. 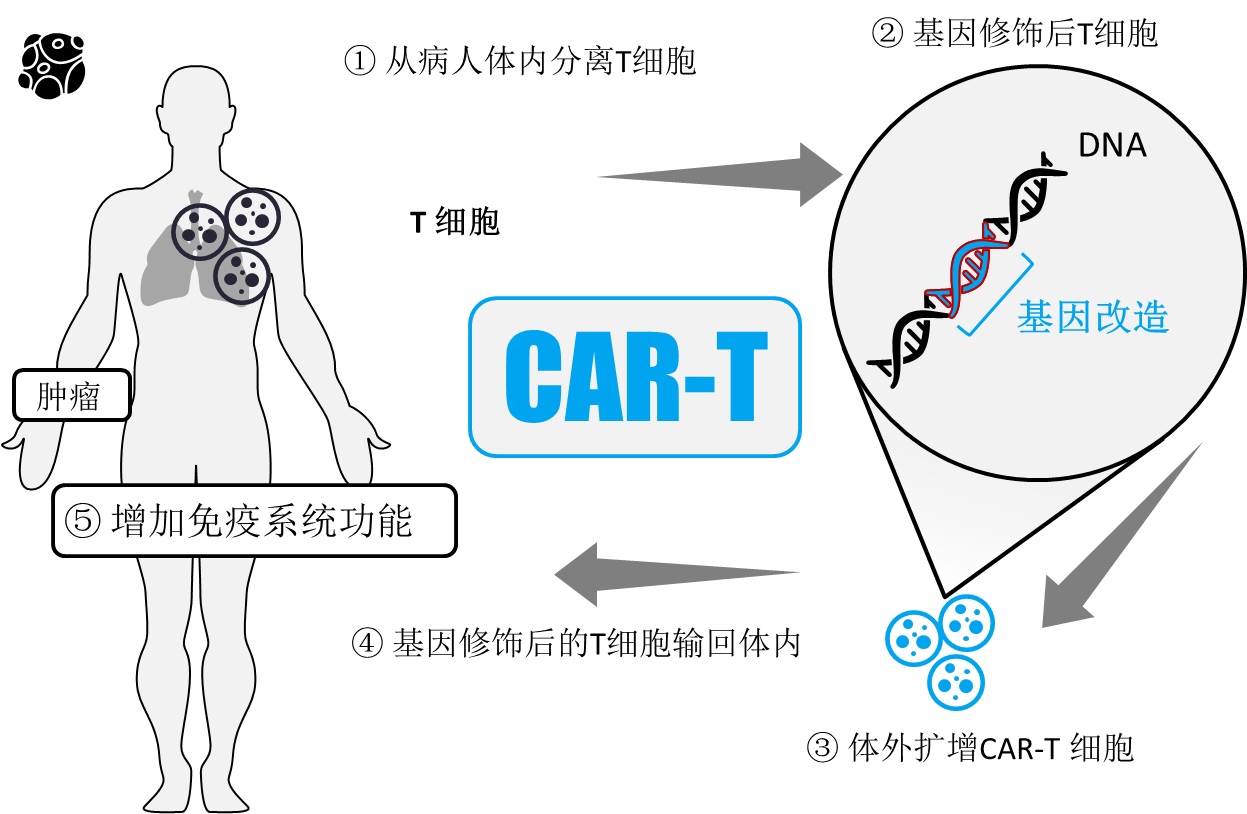
"Gossip" 4 : Where is the magic of CAR-T therapy?
According to reports, there were 30 leukemia patients who were first treated with CAR-T. They have tried various possible treatments, including chemotherapy and targeted therapy. Among them, 15 even underwent bone marrow transplantation, but they failed. Usually, their survival time cannot exceed half a year. According to our Chinese, dead horses are regarded as living horse doctors. They became the first people to eat the crab of CAR-T. As a result, the people shocked the world: the cancer cells of 27 patients completely disappeared after treatment! Twenty patients were reviewed after half a year, and no cancer cells were found!
Currently, whether in China or the United States, CAR-T targets tumor-associated antigens (TAAs) that are not expressed or relatively depleted by most important tissues. Among them, mainly focused on the study of CAR-T cells using CD19, CD20 and CD22 as target antigens for the treatment of B cell tumors.
Taking CD19 as an example, it is expressed not only on the surface of all normal B cells, but also on the surface of most malignant proliferating B cells. In fact, even if treatment with CD19 results in the loss of mature B cells, this side effect is only temporary and recoverable; and since CD19 is not expressed in hematopoietic stem cells, treatment with anti-CD19 CAR-T cells Its toxicity is also limited to B cell aplastic anemia. This "on target/off tumor" toxicity is tolerable in the treatment of B cell leukemia.
Meanwhile, a large number of studies have shown that immunosuppressive effect solid tumor the tumor microenvironment very strong, easy access to the interior aspect of the immune cells of solid tumors; on the other hand, even if the CAR-T cells into the tumor tissue, due to the strong immunosuppressive environment makes it Anti-tumor immune function is also difficult to function properly , which is also an important reason why CAR-T cells are currently mainly used to treat tumors in the blood system. The other targets for research are Her2, GD2, CEA, mesothelin and so on.
"Gossip" 5 : CAR-T has an amazing effect and has become the new favorite of more and more pharmaceutical companies.
The efficacy of CAR-T is so amazing that a large number of drug giants have been involved in the development and clinical transformation of CAR-T, including Novartis, Juno, Kite Pharma, CBMG, and Cellectis. These large pharmaceutical companies already have their own CAR-T products and registered clinical studies. It is worth mentioning that on July 12 this year, the FDA Oncology Expert Advisory Committee (ODAC) unanimously recommended approval with a 10:0 vote. Novartis CAR-T Therapy Tisagenlecleucel (CTL-019) is available. It is said that the FDA will make a final approval decision based on expert opinions before October 3. The indication for CTL-019 is relapsed or refractory children and young adult acute lymphoblastic leukemia (ALL), which means that CTL-019 is very likely to be the world's first approved CAR-T product.
Speaking of it, is it very exciting? However, due to space limitations, our introduction to this issue comes to this point, I want to know what happened, and listen to the next decomposition - CAR-T research and development status . Welcome to continue to pay attention to our columns, we will talk again next time!
References:
1. Curran, Kevin J., Hollie J. Pegram, and Renier J. Brentjens. "Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions." The journal of gene medicine 14.6 (2012): 405-415.
2. The Emily story is from http://emilywhitehead.com/
3. Part of the content, from the book "Cancer, Truth" by Pineapple
Principles and therapeutic applications of CAR-T therapy
What is CAR-T ?
A schematic diagram of the T cell therapy process represented by CAR-T